CARBOHYDRATES:
If you don't know basic chemistry this post will be useless for you don't read it because it waste your time and time is money
The word carbohydrate mean 'hydrates of carbon'mean any compound composed of carbon,hydrogen and oxygen and in which hydrogen and oxygen are found in the same ratio as they are found in the water(2:1), can be called CARBOHYDRATE.This defination of carbohydrate is not perfectly correct because this defination is applicable on many compound but not on all compound . For example formula of glucose is (C6H12O6).If we see glucose formula then we observe that it has caron,hydrogen and oxygen so first condition of above carbohydrate defination is true for it. Now if we observe ratio of hydrogen and oxygen in glucose formula then we found that ratio of hydrogen and oxygen in glucose is same as they are found in water that is 2:1 mean hydrogen is present in double amount then oxygen so according to above defination of carbohydrate glucose is carbohydrate and in real glucose is carbohydrate similarly many other compound like sucrose(C12H22O11) are carbohydrate according to above defination and in reallity. But the problem made when scientist observe that some compound contain carbon,hydrogen and oxygen and the ratio of hydrogen and oxygen is same as they are found in water that is 2:1 but they are not carbohydrate such as acetic acid(C2H4O2) AND lactic acid(C3H6O3) In these two examples you clearly see that they contain carbon,hydrogen and oxygen and ratio of carbon and oxygen is same as they are found in water that is 2:1 so you think them carbohdrate but when they practically obserbved it is found that they are not carbohydrate so it mean above defination of carbohydrate is defective one more defect also present in this defination which is, it was obsebved that some compound contain carbon,hydrogen and oxygen but the ratio of hydrogen and oxygen is not same as they are found in water that is2:1 such as as Rhamnose (C6H12O5) so according to above defination of carbohydrate it is not carbohydrate but when practically obsebve this compound will be a carbohydrate so above defination of carbohydrate is defective so scientist made new defination of carbohydrate which is perfectly correct
" Organic compounds that are polyhydroxy aldehydes or polyhydroxy ketones, or change to such substances on simple chemical transformations as hydrolysis,oxidation, or reduction are known as carbohydrate"
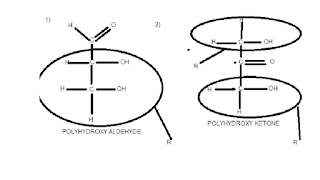
Poly mean many.Aldehydes and ketone are functional groups.As you see i draw aldehyde and ketone functionla group, in aldehde you see one R group attached to one side of carbon and in ketone 2 R group attach to both side of carbon.
This R has diffrent structure .
You understand that how R has diffrent structure from the following rough sketch. As u clearly see in these both sketch that in first sketch R value is C2H3OH and in sketch 2 R value is CH2OH
Oxidation is loss of electron. Reduction is defined as gain of electron.Hydrolysis is breakdown of water in the presence of comlex organic compound not simply breakdown of water but many people say this
thanks for reading if found helpful share it
If you don't know basic chemistry this post will be useless for you don't read it because it waste your time and time is money
The word carbohydrate mean 'hydrates of carbon'mean any compound composed of carbon,hydrogen and oxygen and in which hydrogen and oxygen are found in the same ratio as they are found in the water(2:1), can be called CARBOHYDRATE.This defination of carbohydrate is not perfectly correct because this defination is applicable on many compound but not on all compound . For example formula of glucose is (C6H12O6).If we see glucose formula then we observe that it has caron,hydrogen and oxygen so first condition of above carbohydrate defination is true for it. Now if we observe ratio of hydrogen and oxygen in glucose formula then we found that ratio of hydrogen and oxygen in glucose is same as they are found in water that is 2:1 mean hydrogen is present in double amount then oxygen so according to above defination of carbohydrate glucose is carbohydrate and in real glucose is carbohydrate similarly many other compound like sucrose(C12H22O11) are carbohydrate according to above defination and in reallity. But the problem made when scientist observe that some compound contain carbon,hydrogen and oxygen and the ratio of hydrogen and oxygen is same as they are found in water that is 2:1 but they are not carbohydrate such as acetic acid(C2H4O2) AND lactic acid(C3H6O3) In these two examples you clearly see that they contain carbon,hydrogen and oxygen and ratio of carbon and oxygen is same as they are found in water that is 2:1 so you think them carbohdrate but when they practically obserbved it is found that they are not carbohydrate so it mean above defination of carbohydrate is defective one more defect also present in this defination which is, it was obsebved that some compound contain carbon,hydrogen and oxygen but the ratio of hydrogen and oxygen is not same as they are found in water that is2:1 such as as Rhamnose (C6H12O5) so according to above defination of carbohydrate it is not carbohydrate but when practically obsebve this compound will be a carbohydrate so above defination of carbohydrate is defective so scientist made new defination of carbohydrate which is perfectly correct
" Organic compounds that are polyhydroxy aldehydes or polyhydroxy ketones, or change to such substances on simple chemical transformations as hydrolysis,oxidation, or reduction are known as carbohydrate"
Poly mean many.Aldehydes and ketone are functional groups.As you see i draw aldehyde and ketone functionla group, in aldehde you see one R group attached to one side of carbon and in ketone 2 R group attach to both side of carbon.
This R has diffrent structure .
You understand that how R has diffrent structure from the following rough sketch. As u clearly see in these both sketch that in first sketch R value is C2H3OH and in sketch 2 R value is CH2OH
Oxidation is loss of electron. Reduction is defined as gain of electron.Hydrolysis is breakdown of water in the presence of comlex organic compound not simply breakdown of water but many people say this
thanks for reading if found helpful share it

Comments
Post a Comment